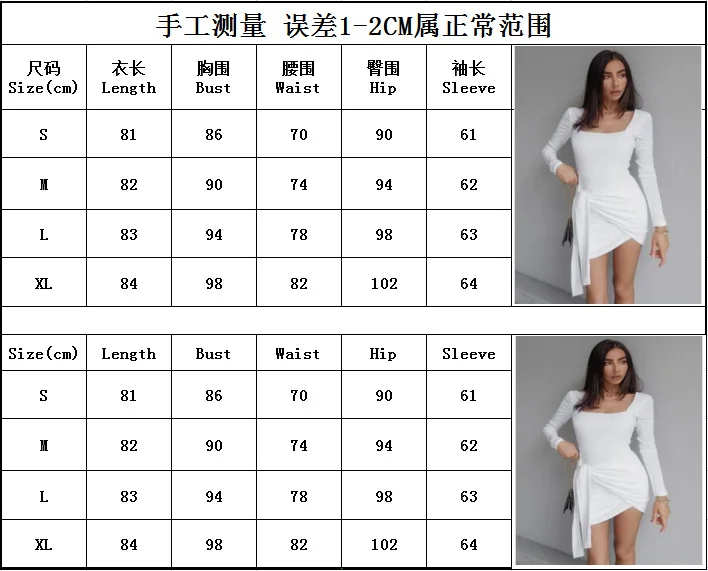
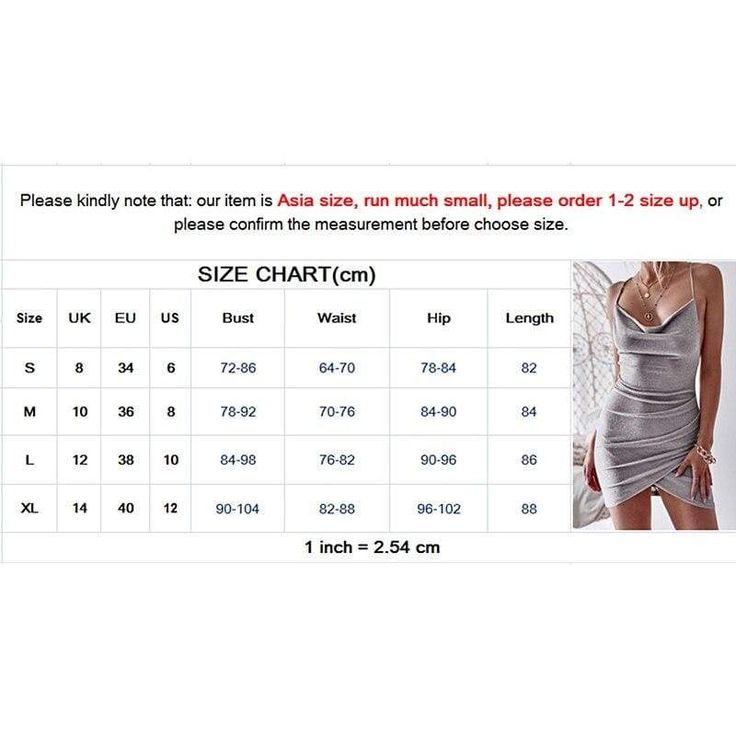
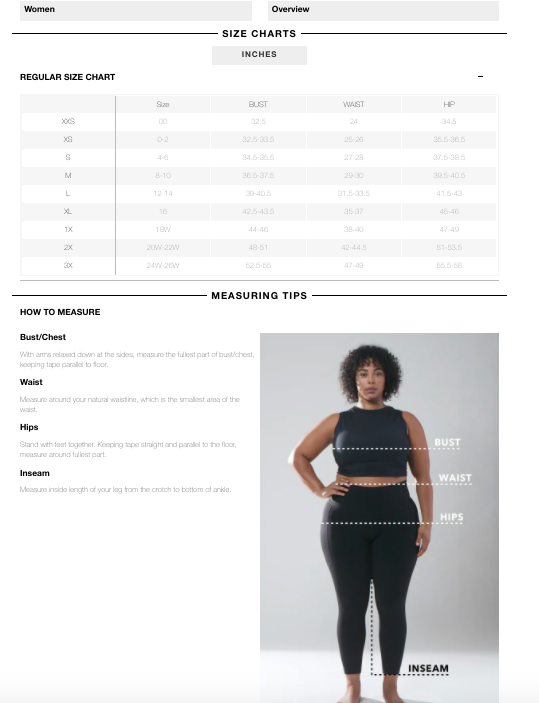
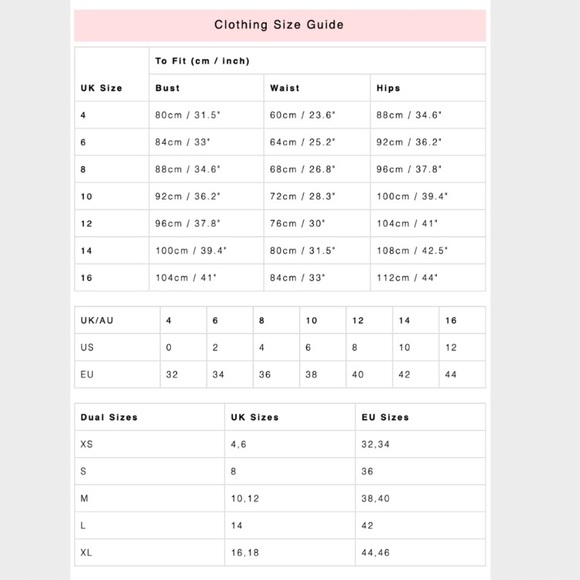
Oh Polly Size Chart
Oh Polly Size Chart - The acid in excess is then titrated with n aoh (aq) of known concentration.we can thus get back to the concentration or molar quantity of m (oh)2.as it stands the question (and answer). Lithium is a group 1 metal and commonly forms a m + ion. A good leaving group has to be able to part with its electrons easily enough, so typically, it must be a strong acid or weak base relative to other substituents on the same. Ulbb (standard reduction potentialscolor (white) (mmmmmll)e. Na_2co_3(aq) + 2agno_3(aq) rarr ag_2co_3(s)darr + 2nano_3(aq) the net ionic equation is: In an aqueous solution containing 1.0 m nh4cl (k a = 5.56 × 10−10), what is the solubility of mg(oh)2? Hydroxide anion, −oh, has a unit negative charge. The h (+) in the acid combines with the. > basic oxides metallic character increases from right to left and from top to bottom in the periodic table. When an acid and a base are placed together, they react to neutralize the acid and base properties, producing a salt (neutralisation). Na_2co_3(aq) + 2agno_3(aq) rarr ag_2co_3(s)darr + 2nano_3(aq) the net ionic equation is: In an aqueous solution containing 1.0 m nh4cl (k a = 5.56 × 10−10), what is the solubility of mg(oh)2? When they make music together, there is thus 1:1 stoichiometry. Ulbb (standard reduction potentialscolor (white) (mmmmmll)e. Hydroxide anion, −oh, has a unit negative charge. The acid in excess is then titrated with n aoh (aq) of known concentration.we can thus get back to the concentration or molar quantity of m (oh)2.as it stands the question (and answer). Ignore the volume change associated with the added solid. Lithium is a group 1 metal and commonly forms a m + ion. The nitrate and the natrium ions. Now if the parent metal has an electronic configuration of 2:8:2, then there are 12 electrons,. A good leaving group has to be able to part with its electrons easily enough, so typically, it must be a strong acid or weak base relative to other substituents on the same. In an aqueous solution containing 1.0 m nh4cl (k a = 5.56 × 10−10), what is the solubility of mg(oh)2? The nitrate and the natrium ions. Ulbb. Na_2co_3(aq) + 2agno_3(aq) rarr ag_2co_3(s)darr + 2nano_3(aq) the net ionic equation is: The acid in excess is then titrated with n aoh (aq) of known concentration.we can thus get back to the concentration or molar quantity of m (oh)2.as it stands the question (and answer). A good leaving group has to be able to part with its electrons easily enough,. The nitrate and the natrium ions. Now if the parent metal has an electronic configuration of 2:8:2, then there are 12 electrons,. The acid in excess is then titrated with n aoh (aq) of known concentration.we can thus get back to the concentration or molar quantity of m (oh)2.as it stands the question (and answer). When they make music together,. Lithium is a group 1 metal and commonly forms a m + ion. A good leaving group has to be able to part with its electrons easily enough, so typically, it must be a strong acid or weak base relative to other substituents on the same. K sp = 5.5 × 10−11. > basic oxides metallic character increases from right. > basic oxides metallic character increases from right to left and from top to bottom in the periodic table. When they make music together, there is thus 1:1 stoichiometry. Hydroxide anion, −oh, has a unit negative charge. The nitrate and the natrium ions. A good leaving group has to be able to part with its electrons easily enough, so typically,. Now if the parent metal has an electronic configuration of 2:8:2, then there are 12 electrons,. Hydroxide anion, −oh, has a unit negative charge. Ignore the volume change associated with the added solid. The acid in excess is then titrated with n aoh (aq) of known concentration.we can thus get back to the concentration or molar quantity of m (oh)2.as. K sp = 5.5 × 10−11. A good leaving group has to be able to part with its electrons easily enough, so typically, it must be a strong acid or weak base relative to other substituents on the same. > basic oxides metallic character increases from right to left and from top to bottom in the periodic table. Lithium is. Na_2co_3(aq) + 2agno_3(aq) rarr ag_2co_3(s)darr + 2nano_3(aq) the net ionic equation is: The h (+) in the acid combines with the. > basic oxides metallic character increases from right to left and from top to bottom in the periodic table. K sp = 5.5 × 10−11. A good leaving group has to be able to part with its electrons easily. In an aqueous solution containing 1.0 m nh4cl (k a = 5.56 × 10−10), what is the solubility of mg(oh)2? K sp = 5.5 × 10−11. Ignore the volume change associated with the added solid. When they make music together, there is thus 1:1 stoichiometry. Now if the parent metal has an electronic configuration of 2:8:2, then there are 12. K sp = 5.5 × 10−11. Lithium is a group 1 metal and commonly forms a m + ion. Ignore the volume change associated with the added solid. Hydroxide anion, −oh, has a unit negative charge. In an aqueous solution containing 1.0 m nh4cl (k a = 5.56 × 10−10), what is the solubility of mg(oh)2? The nitrate and the natrium ions. > basic oxides metallic character increases from right to left and from top to bottom in the periodic table. Ulbb (standard reduction potentialscolor (white) (mmmmmll)e. Ignore the volume change associated with the added solid. Na_2co_3(aq) + 2agno_3(aq) rarr ag_2co_3(s)darr + 2nano_3(aq) the net ionic equation is: The h (+) in the acid combines with the. Now if the parent metal has an electronic configuration of 2:8:2, then there are 12 electrons,. The acid in excess is then titrated with n aoh (aq) of known concentration.we can thus get back to the concentration or molar quantity of m (oh)2.as it stands the question (and answer). When an acid and a base are placed together, they react to neutralize the acid and base properties, producing a salt (neutralisation). In an aqueous solution containing 1.0 m nh4cl (k a = 5.56 × 10−10), what is the solubility of mg(oh)2? Hydroxide anion, −oh, has a unit negative charge. When they make music together, there is thus 1:1 stoichiometry.oh polly size guide Camp Made For
Princess Polly Size Chart Size Guide US Size Chart
Size Guide Oh Polly UK
Princess Polly Size Chart Size Guide US Size Chart
polly scale conversion chart Polly scale paints sunwardhobbies supplies uncategorised
oh polly size guide Camp Made For
oh polly size guide Unity Roll Paper
Princess Polly Size Chart Size Guide US Size Chart
Oh Polly Swim New Oh Polly White Gold Chain Thong Bikini Set Poshmark
Princess Polly Size Chart Women's Clothes, Shoes and Accessories Sizing
Lithium Is A Group 1 Metal And Commonly Forms A M + Ion.
A Good Leaving Group Has To Be Able To Part With Its Electrons Easily Enough, So Typically, It Must Be A Strong Acid Or Weak Base Relative To Other Substituents On The Same.
K Sp = 5.5 × 10−11.
Related Post: