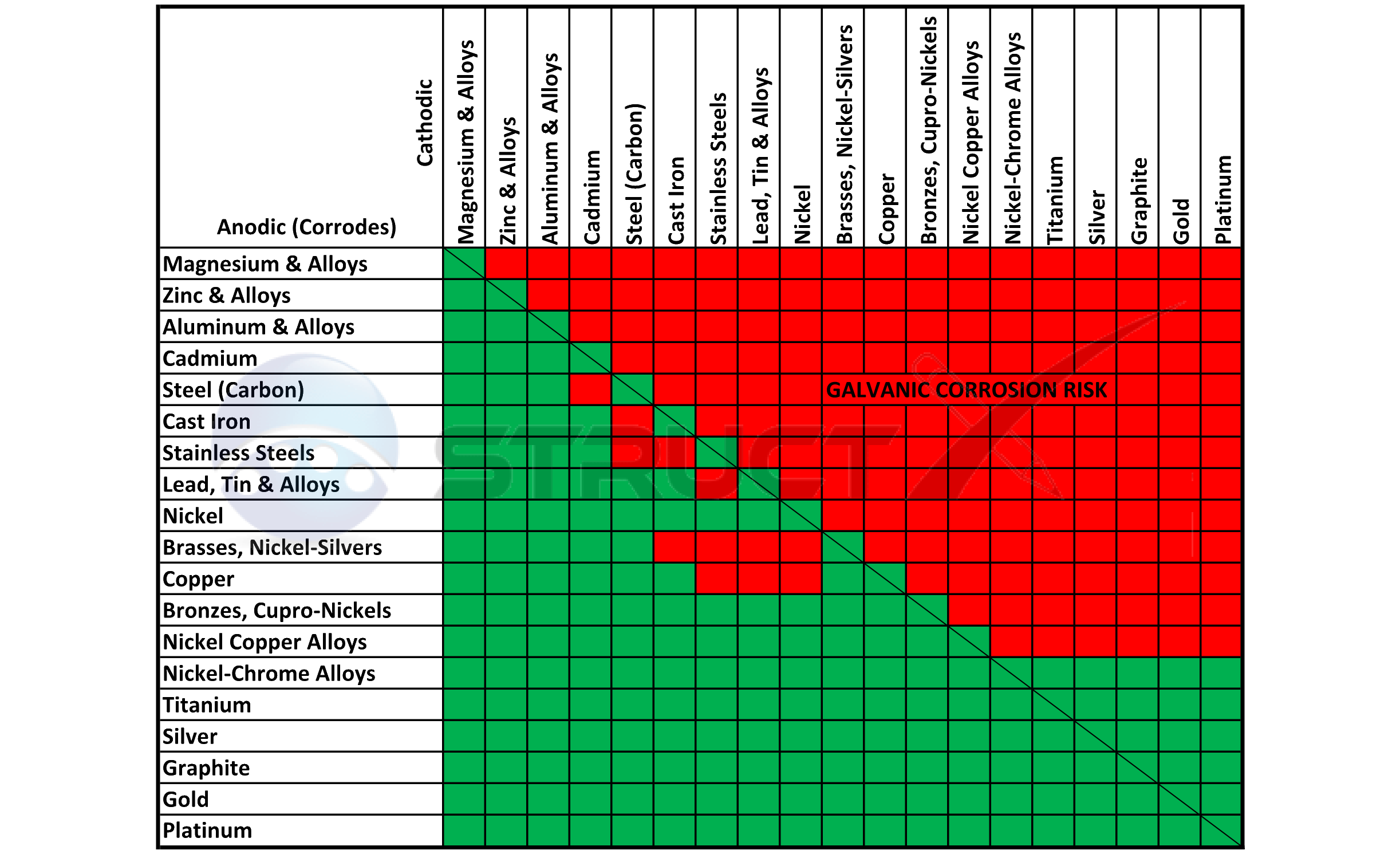
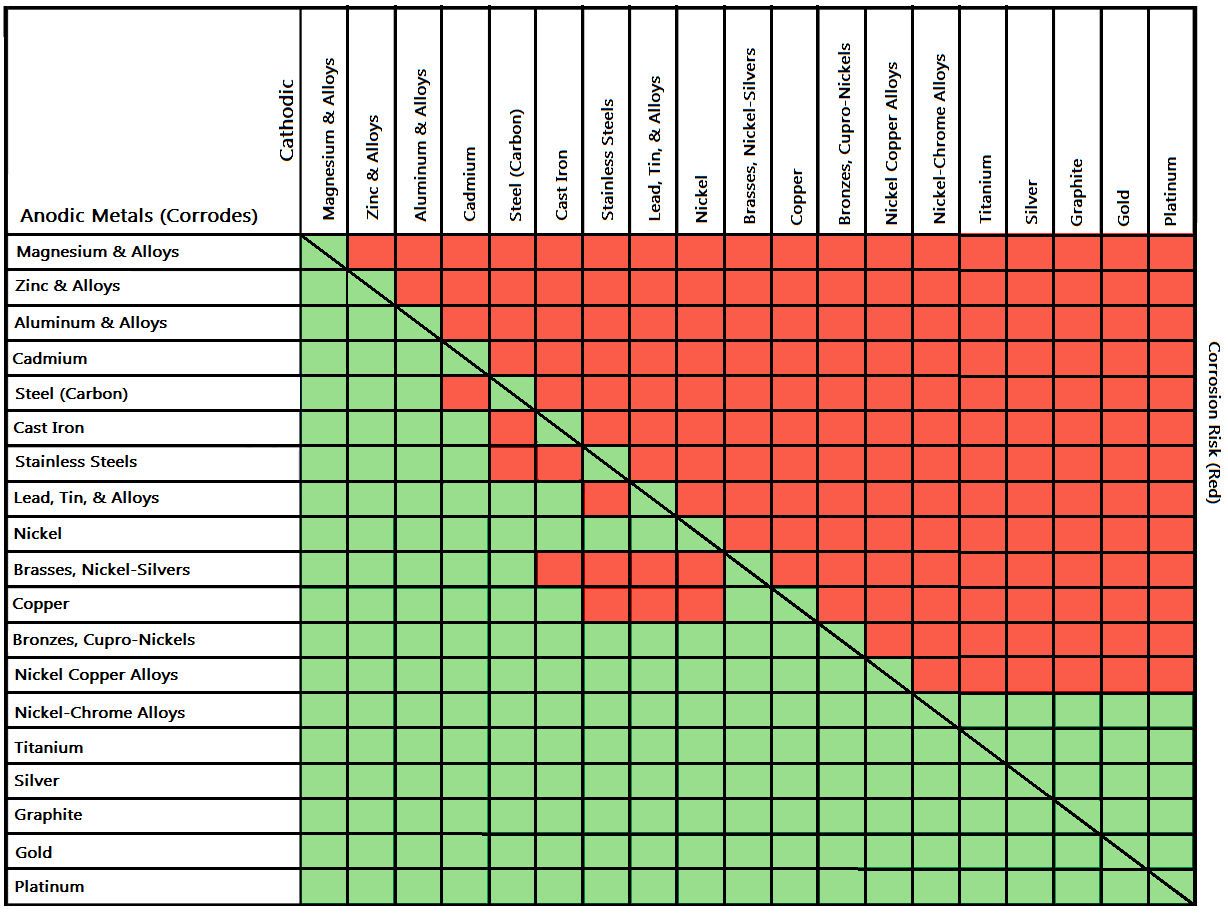
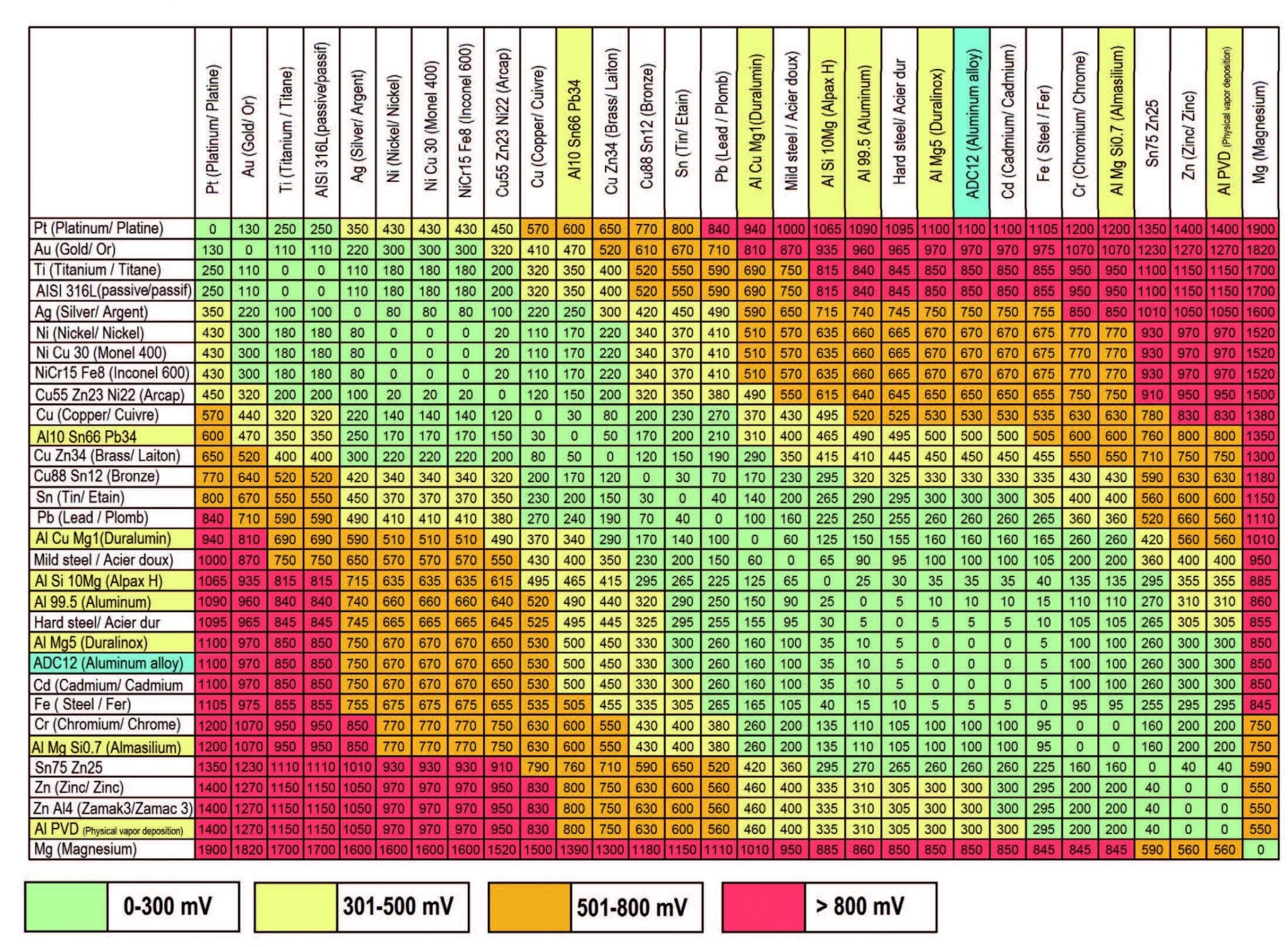
Galvanic Corrosion Chart
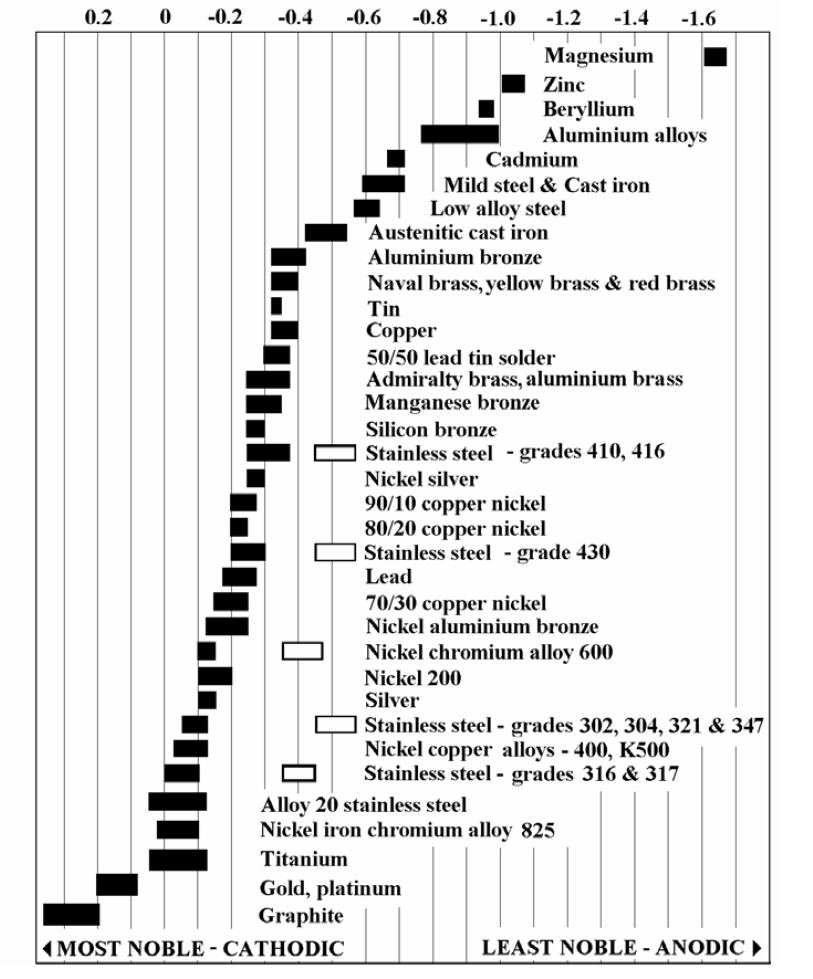
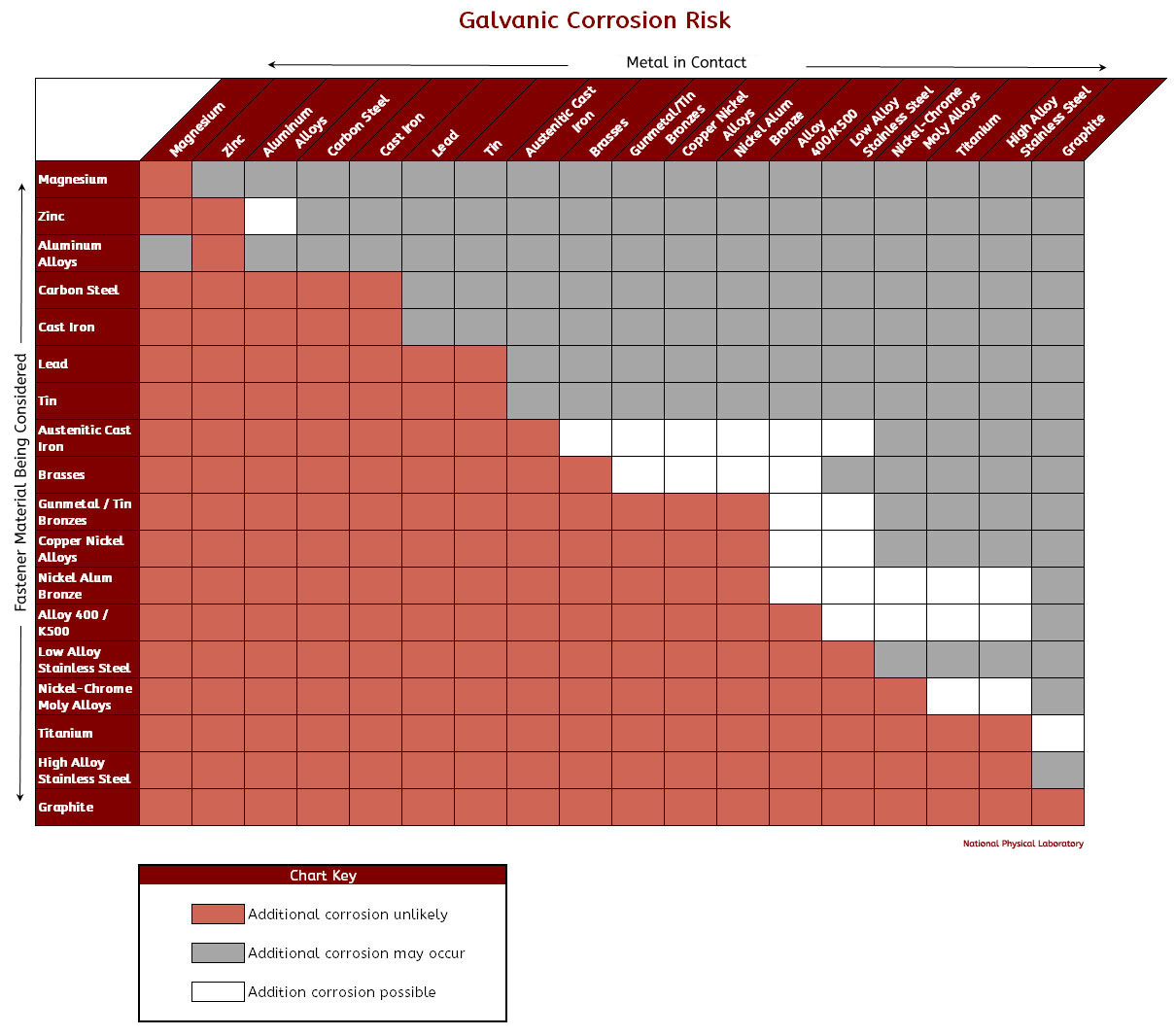
Galvanic Corrosion Chart - There are three conditions that must. These charts show which commonly used metals are compatible and which will result in galvanic corrosion when in contact. This galvanic reaction chart for dissimilar metals is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal coming into contact with other metals. An anode, cathode, electrolyte, and return path. A typical rule of thumb is that voltage differences. For galvanic corrosion to occur, four elements are necessary: Below is a galvanic reaction chart for dissimilar metals. Ac43.13, starting at par 247, briefly covers several types of corrosion and corrosion protection. Below, we give a brief overview of galvanic corrosion and provide a galvanic corrosion chart to help fabricators and machinists avoid using the wrong metal combinations. The galvanic series chart below shows metals and their electrochemical voltage range (relative activity in flowing sea water). For galvanic corrosion to occur, four elements are necessary: Ac43.13, starting at par 247, briefly covers several types of corrosion and corrosion protection. Galvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact with each other oxidizes or corrodes. This galvanic reaction chart for dissimilar metals is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal coming into contact with other metals. When design requires that dissimilar metals come in contact, galvanic compatibility can be managed by finishes and plating which protects the base materials from corrosion. Below, we give a brief overview of galvanic corrosion and provide a galvanic corrosion chart to help fabricators and machinists avoid using the wrong metal combinations. The galvanic corrosion process is a transfer of electrons between two electrodes. Below is a galvanic reaction chart for dissimilar metals. The grouping of materials is an early method of ms33586 which was superseded in 1969 by. These charts show which commonly used metals are compatible and which will result in galvanic corrosion when in contact. Ac43.13, starting at par 247, briefly covers several types of corrosion and corrosion protection. This chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal coming into contact with. This galvanic reaction chart for dissimilar metals is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given. When design requires that dissimilar metals come in contact, galvanic compatibility can be managed by finishes and plating which protects the base materials from corrosion. These charts show which commonly used metals are compatible and which will result in galvanic corrosion when in contact. This chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with. There are three conditions that must. These charts show which commonly used metals are compatible and which will result in galvanic corrosion when in contact. The galvanic series chart below shows metals and their electrochemical voltage range (relative activity in flowing sea water). The galvanic corrosion process is a transfer of electrons between two electrodes. An anode, cathode, electrolyte, and. An anode, cathode, electrolyte, and return path. Galvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact with each other oxidizes or corrodes. A typical rule of thumb is that voltage differences. The galvanic series chart below shows metals and their electrochemical voltage range (relative activity in flowing sea water). The galvanic corrosion. Below is a galvanic reaction chart for dissimilar metals. For galvanic corrosion to occur, four elements are necessary: When design requires that dissimilar metals come in contact, galvanic compatibility can be managed by finishes and plating which protects the base materials from corrosion. This galvanic reaction chart for dissimilar metals is designed to assist in broadly assessing the risk of. An anode, cathode, electrolyte, and return path. The grouping of materials is an early method of ms33586 which was superseded in 1969 by. A typical rule of thumb is that voltage differences. For galvanic corrosion to occur, four elements are necessary: This galvanic reaction chart for dissimilar metals is designed to assist in broadly assessing the risk of galvanic corrosion. The galvanic series chart below shows metals and their electrochemical voltage range (relative activity in flowing sea water). This galvanic reaction chart for dissimilar metals is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal coming into contact with other metals. The galvanic corrosion process is a transfer of electrons between two electrodes.. This chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal coming into contact with. A typical rule of thumb is that voltage differences. There are three conditions that must. Galvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact with each other oxidizes or. A typical rule of thumb is that voltage differences. The galvanic corrosion process is a transfer of electrons between two electrodes. Galvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact with each other oxidizes or corrodes. Below is a galvanic reaction chart for dissimilar metals. This chart is designed to assist in. These charts show which commonly used metals are compatible and which will result in galvanic corrosion when in contact. This galvanic reaction chart for dissimilar metals is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal coming into contact with other metals. Galvanic corrosion (some times called dissimilar metal corrosion) is the process. For galvanic corrosion to occur, four elements are necessary: The galvanic series chart below shows metals and their electrochemical voltage range (relative activity in flowing sea water). There are three conditions that must. Galvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact with each other oxidizes or corrodes. Below is a galvanic reaction chart for dissimilar metals. An anode, cathode, electrolyte, and return path. These charts show which commonly used metals are compatible and which will result in galvanic corrosion when in contact. When design requires that dissimilar metals come in contact, galvanic compatibility can be managed by finishes and plating which protects the base materials from corrosion. Ac43.13, starting at par 247, briefly covers several types of corrosion and corrosion protection. The grouping of materials is an early method of ms33586 which was superseded in 1969 by. A typical rule of thumb is that voltage differences. The galvanic corrosion process is a transfer of electrons between two electrodes.Galvanic Corrosion Chart Dissimilar Metals A Visual Reference of Charts Chart Master
Galvanic Action Corrosion Prevention Architect's Blog
Stainless Steel Galvanic Corrosion Chart
Galvanic Corrosion [with Chart] EngineerExcel
Galvanic Series (electrochemical series)
Galvanic Potential Chart Galvanic Corrosion Potential Chart Galvanic Corrosion Chart
Galvanic Corrosion SSINA
Galvanic Corrosion Chart PDF Corrosion Electrochemistry
Stainless Steel Galvanic Corrosion Chart
Galvanic Corrosion Chart
This Chart Is Designed To Assist In Broadly Assessing The Risk Of Galvanic Corrosion Associated With A Given Metal Coming Into Contact With.
This Galvanic Reaction Chart For Dissimilar Metals Is Designed To Assist In Broadly Assessing The Risk Of Galvanic Corrosion Associated With A Given Metal Coming Into Contact With Other Metals.
Below, We Give A Brief Overview Of Galvanic Corrosion And Provide A Galvanic Corrosion Chart To Help Fabricators And Machinists Avoid Using The Wrong Metal Combinations.
Related Post:


![Galvanic Corrosion [with Chart] EngineerExcel](https://engineerexcel.com/wp-content/uploads/2023/03/galvanic-corrosion-chart.png)