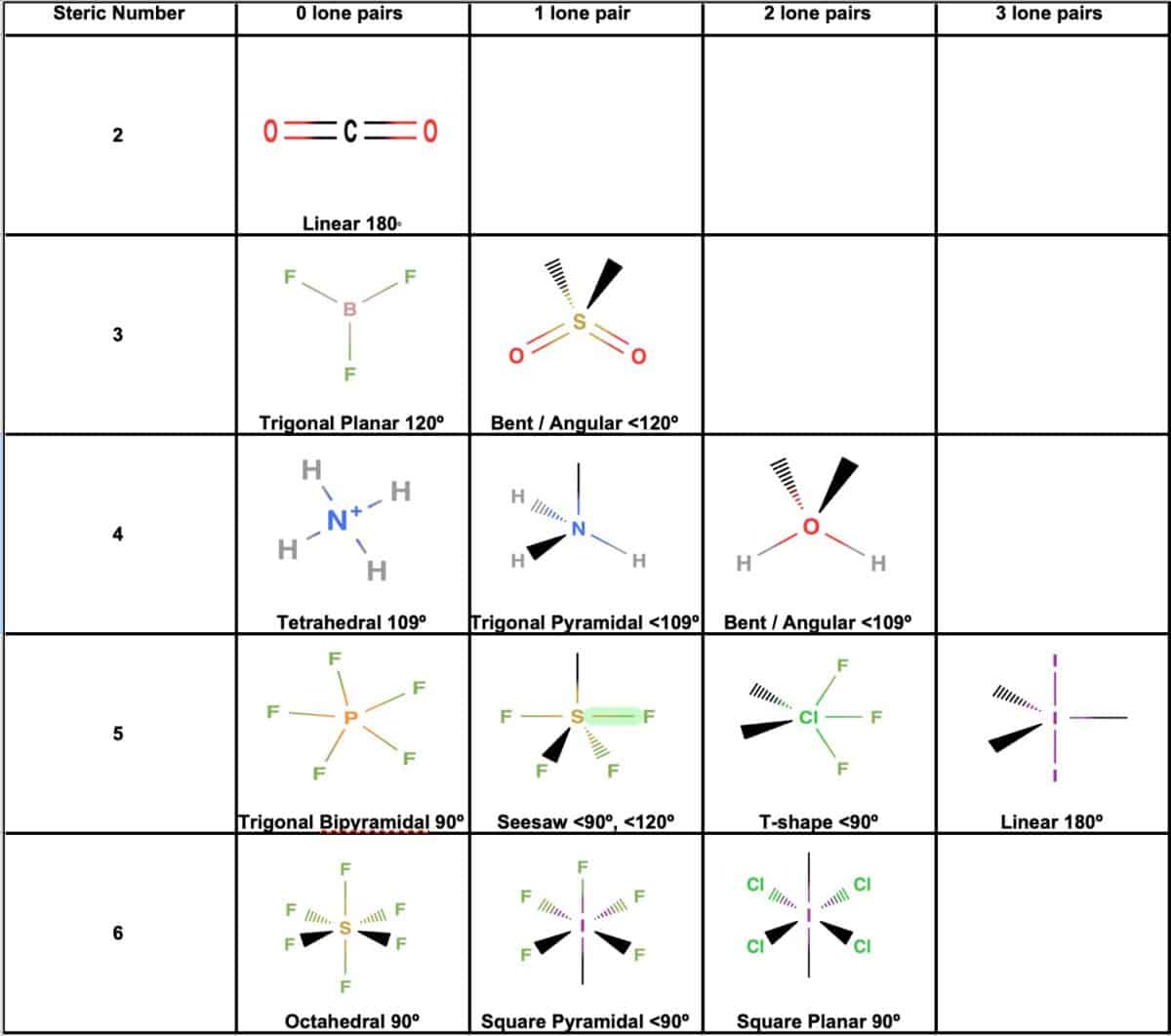
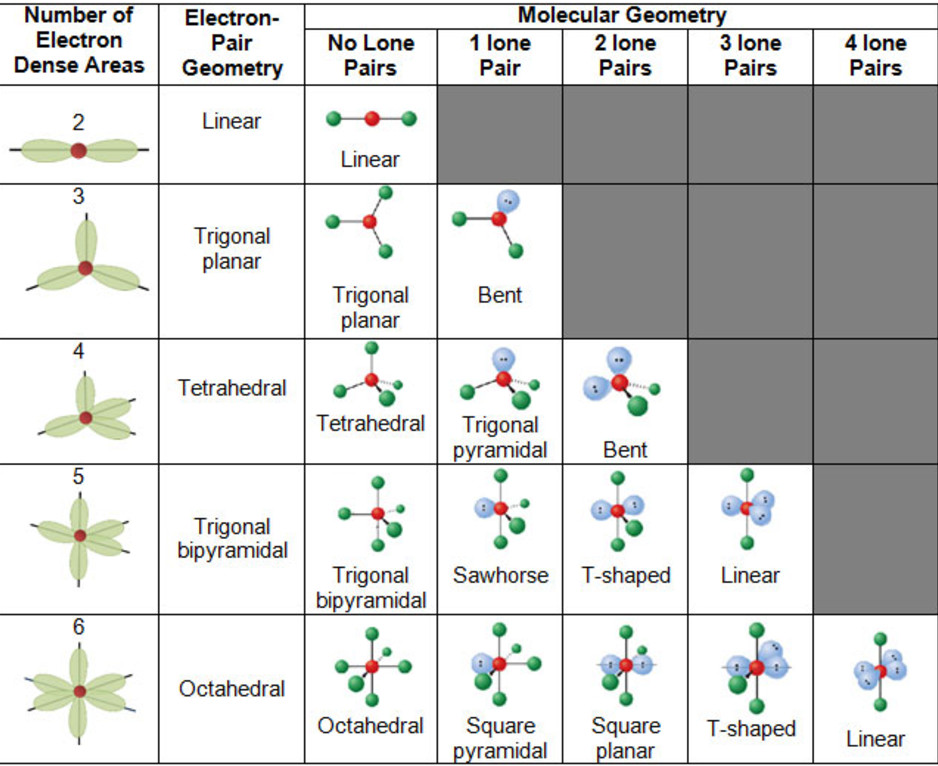
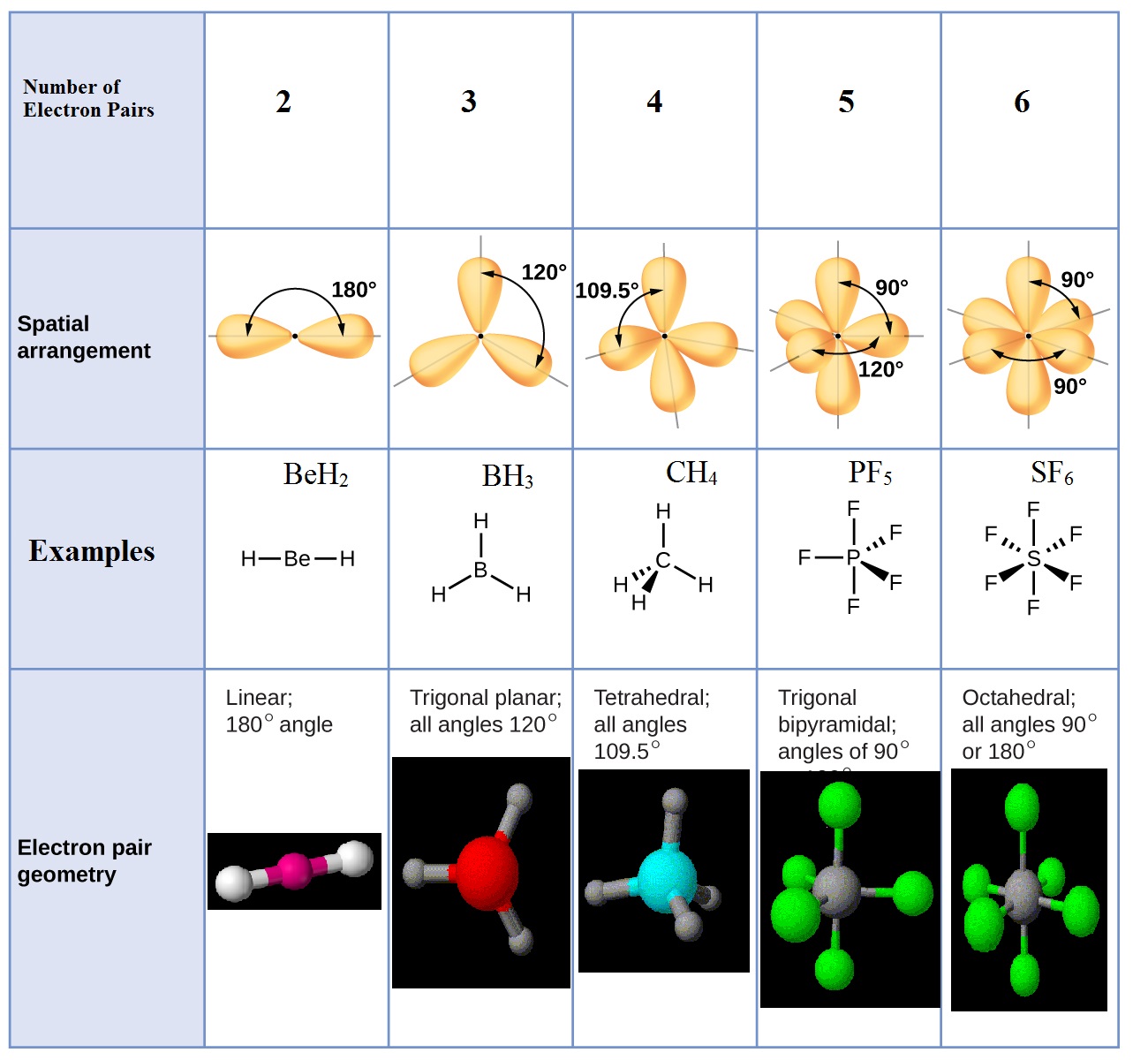
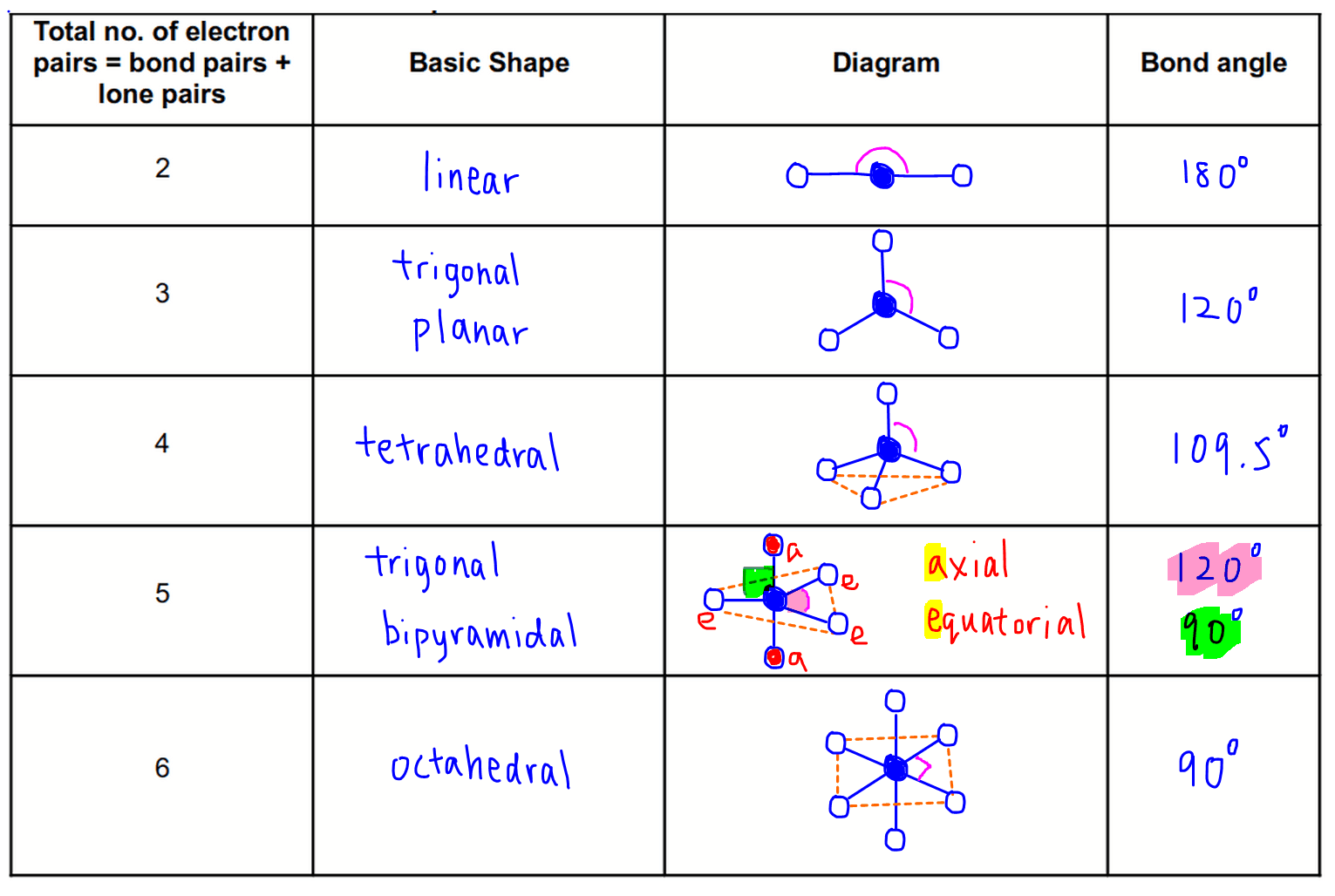
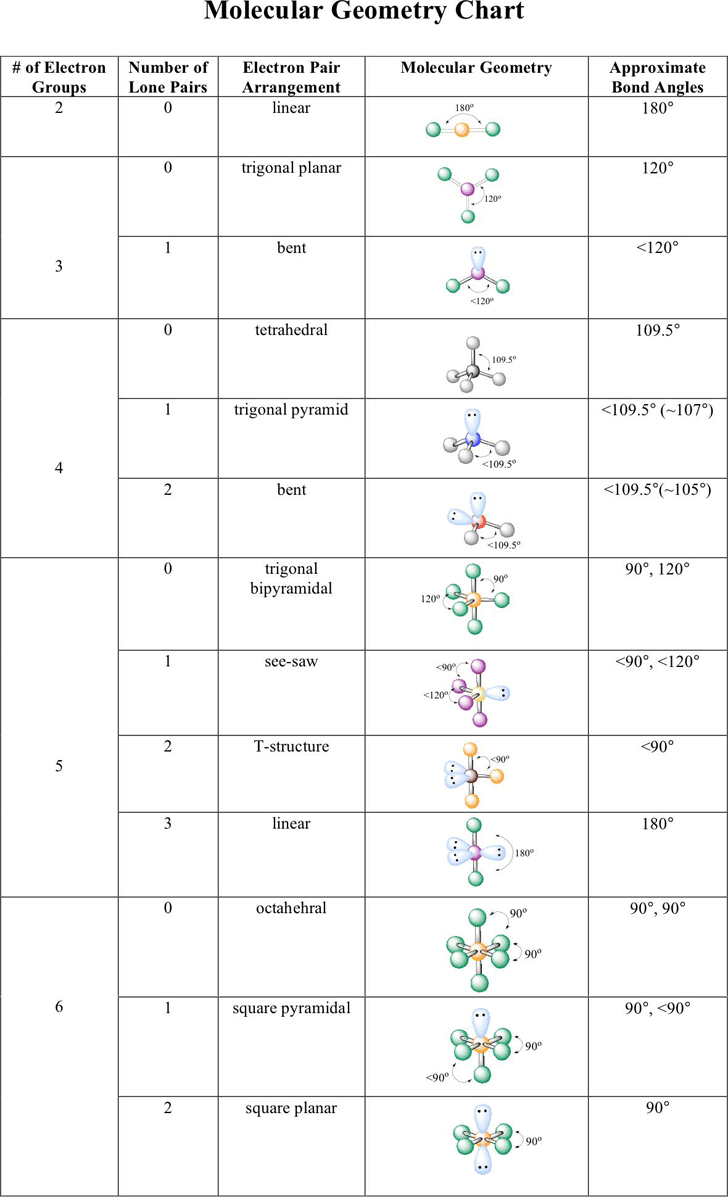
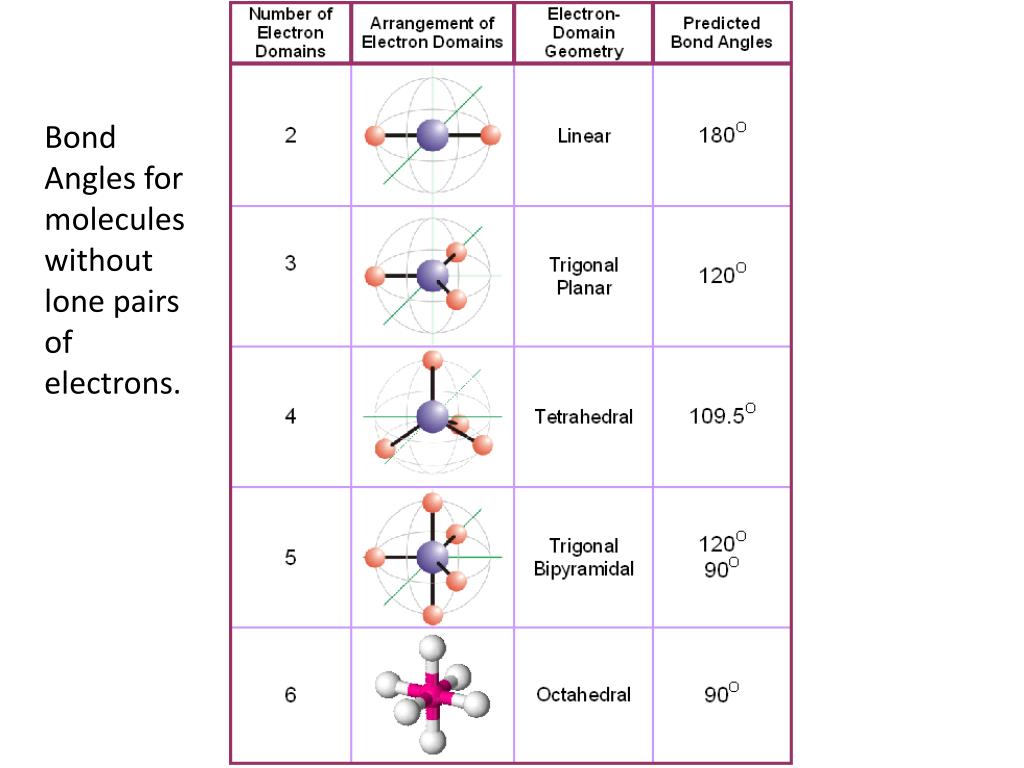
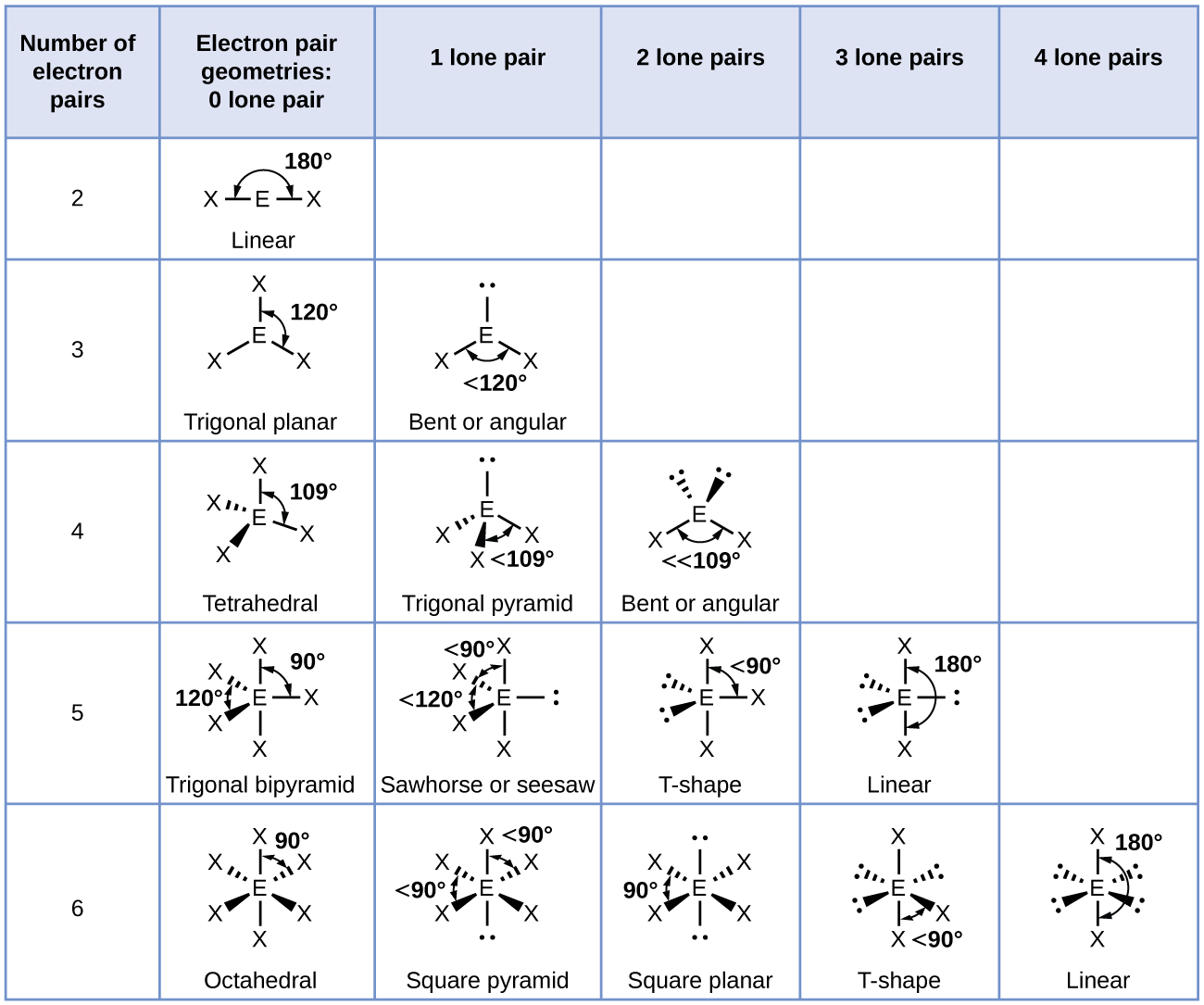
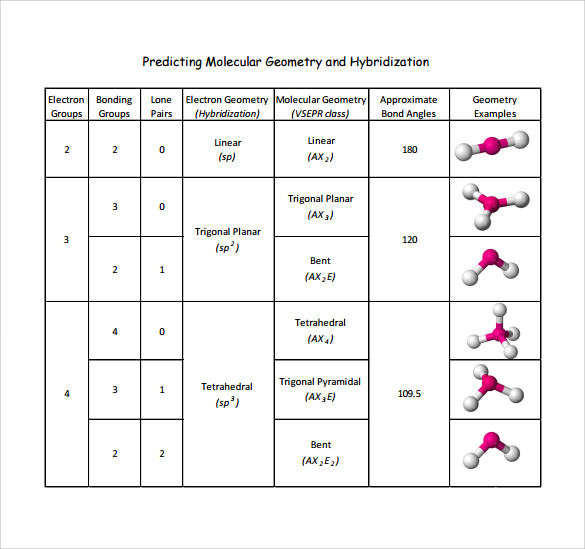
Electronic And Molecular Geometry Chart
Electronic And Molecular Geometry Chart - What are the different shapes of molecules. Molecular geometry, on the other hand, helps us in determining the entire atom and its configuration. Explore our table of common electron geometries with bonding domains, bond angles, and formulas. There is a three step approach to determining the geometry of a molecule. Abnem a = central atom, b = directly bonded atoms to a, and e = nonbonding (unshared) pairs of electrons *note that a molecule formed by joining only two (2). Determine the lewis dot structure of the compound. A group of electrons is. Here we will discuss both electron pair geometries (also called electron group or electron domain geometries) and molecular geometries (also called molecular shapes). Check out a table of molecular geometries with examples and diagrams. In chemistry, vsepr theory is based on the principle that each atom in a molecule will seek a geometry that maximizes the distance between valence electron pairs, thus minimizing. Here we will discuss both electron pair geometries (also called electron group or electron domain geometries) and molecular geometries (also called molecular shapes). Determine the electron geometry from the lewis dot structure. In chemistry, vsepr theory is based on the principle that each atom in a molecule will seek a geometry that maximizes the distance between valence electron pairs, thus minimizing. Molecular geometry, on the other hand, helps us in determining the entire atom and its configuration. There is a three step approach to determining the geometry of a molecule. Students and scientists can use these charts to create three. A molecular geometry chart is a collection of rules on how molecules and electrons will connect and shape a molecule. Determine the lewis dot structure of the compound. Abnem a = central atom, b = directly bonded atoms to a, and e = nonbonding (unshared) pairs of electrons *note that a molecule formed by joining only two (2). Check out a table of molecular geometries with examples and diagrams. A molecular geometry chart is a collection of rules on how molecules and electrons will connect and shape a molecule. Determine the electron geometry from the lewis dot structure. In chemistry, vsepr theory is based on the principle that each atom in a molecule will seek a geometry that maximizes the distance between valence electron pairs, thus minimizing. What are. You can manipulate the number of single, double, or triple bonds and lone. A group of electrons is. Students and scientists can use these charts to create three. Abnem a = central atom, b = directly bonded atoms to a, and e = nonbonding (unshared) pairs of electrons *note that a molecule formed by joining only two (2). Check out. Explore our table of common electron geometries with bonding domains, bond angles, and formulas. You can manipulate the number of single, double, or triple bonds and lone. What are the different shapes of molecules. Electron geometry helps us in determining the arrangement of various electron groups. Determine the lewis dot structure of the compound. Explore our table of common electron geometries with bonding domains, bond angles, and formulas. In chemistry, vsepr theory is based on the principle that each atom in a molecule will seek a geometry that maximizes the distance between valence electron pairs, thus minimizing. Abnem a = central atom, b = directly bonded atoms to a, and e = nonbonding (unshared). A group of electrons is. There is a three step approach to determining the geometry of a molecule. Here we will discuss both electron pair geometries (also called electron group or electron domain geometries) and molecular geometries (also called molecular shapes). Explore our table of common electron geometries with bonding domains, bond angles, and formulas. In chemistry, vsepr theory is. A group of electrons is. Check out a table of molecular geometries with examples and diagrams. Abnem a = central atom, b = directly bonded atoms to a, and e = nonbonding (unshared) pairs of electrons *note that a molecule formed by joining only two (2). Explore our table of common electron geometries with bonding domains, bond angles, and formulas.. A group of electrons is. Determine the electron geometry from the lewis dot structure. A molecular geometry chart is a collection of rules on how molecules and electrons will connect and shape a molecule. Explore our table of common electron geometries with bonding domains, bond angles, and formulas. You can manipulate the number of single, double, or triple bonds and. Abnem a = central atom, b = directly bonded atoms to a, and e = nonbonding (unshared) pairs of electrons *note that a molecule formed by joining only two (2). Students and scientists can use these charts to create three. Explore our table of common electron geometries with bonding domains, bond angles, and formulas. What are the different shapes of. Molecular geometry, on the other hand, helps us in determining the entire atom and its configuration. What are the different shapes of molecules. Students and scientists can use these charts to create three. In chemistry, vsepr theory is based on the principle that each atom in a molecule will seek a geometry that maximizes the distance between valence electron pairs,. Check out a table of molecular geometries with examples and diagrams. Electron geometry helps us in determining the arrangement of various electron groups. Students and scientists can use these charts to create three. Molecular geometry, on the other hand, helps us in determining the entire atom and its configuration. In chemistry, vsepr theory is based on the principle that each. Determine the lewis dot structure of the compound. Molecular geometry, on the other hand, helps us in determining the entire atom and its configuration. Determine the electron geometry from the lewis dot structure. A molecular geometry chart is a collection of rules on how molecules and electrons will connect and shape a molecule. Abnem a = central atom, b = directly bonded atoms to a, and e = nonbonding (unshared) pairs of electrons *note that a molecule formed by joining only two (2). Here we will discuss both electron pair geometries (also called electron group or electron domain geometries) and molecular geometries (also called molecular shapes). Check out a table of molecular geometries with examples and diagrams. There is a three step approach to determining the geometry of a molecule. Students and scientists can use these charts to create three. A group of electrons is. You can manipulate the number of single, double, or triple bonds and lone. In chemistry, vsepr theory is based on the principle that each atom in a molecule will seek a geometry that maximizes the distance between valence electron pairs, thus minimizing.Molecular Geometry Chart and linear molecules
Molecular Geometry Chart and linear molecules
Valence Shell Electron Pair Repulsion Theory Chemical Bonding and Molecular Structure
Electron pair geometry and molecular geometry chart volfplus
Electron vs molecular geometry chart stopfiko
Molecular Geometry Chart Template Free Download Speedy Template
Electron and molecular geometry chart examples heryhd
Electron and molecular geometry chart examples michaelhost
Electron and Molecular Geometries … Molecular geometry, Teaching chemistry, Chemistry lessons
FREE 8+ Sample Molecular Geometry Chart Templates in PDF MS Word
Electron Geometry Helps Us In Determining The Arrangement Of Various Electron Groups.
What Are The Different Shapes Of Molecules.
Explore Our Table Of Common Electron Geometries With Bonding Domains, Bond Angles, And Formulas.
Related Post: