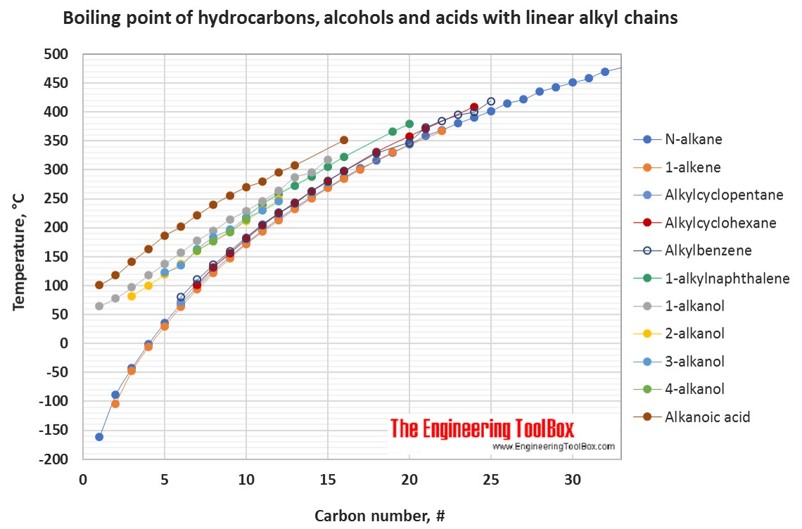
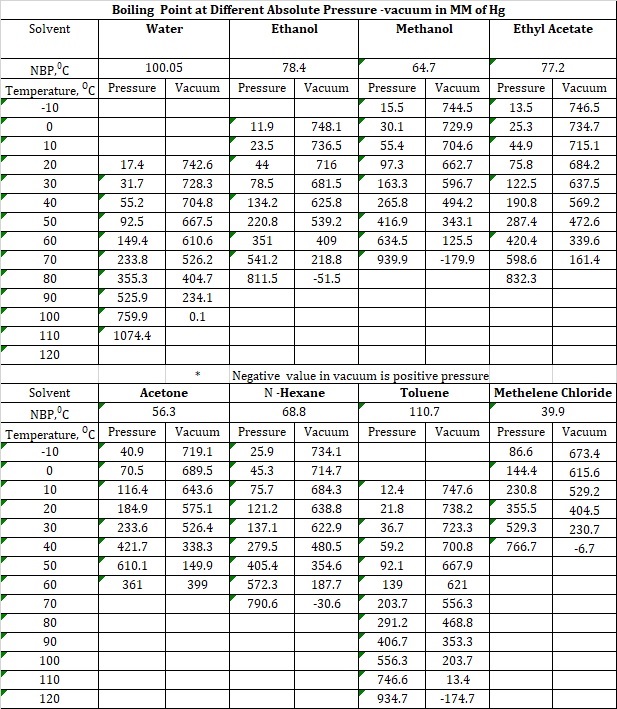
Distillation Temperature Chart
Distillation Temperature Chart - Distillation is a separation technique that is used to extract a mixture of solids in a liquid. Distillation refers to the selective boiling and subsequent condensation of a component in a liquid mixture. Distillation is a separation technique used to separate liquid (the solvent) from a mixture and keep the liquid part. Distillation is a physical separation technique that employs the differences in boiling points of substances to separate components within a liquid mixture. It is a physical separation technique used to separate. It is used to separate liquids from nonvolatile. Distillation is a process that involves separating and purifying the components of a mixture by heating and cooling. Several distillation variations are used in the organic laboratory. Distillation, also classical distillation, is the process of separating the component substances of a liquid mixture of two or more chemically discrete substances; Distillation is a separation technique that separates the liquids upon heating on the basis of their boiling points. Distillation is the process of separating components of a mixture based on different boiling points. Distillation is a separation technique used to separate liquid (the solvent) from a mixture and keep the liquid part. It is used to separate liquids from nonvolatile. After boiling the mixture of liquids the almost pure liquid is. It is a separation technique that can be used to either increase the concentration of a. Distillation is a separation technique that is used to extract a mixture of solids in a liquid. Distillation is a separation technique that separates the liquids upon heating on the basis of their boiling points. Distillation refers to the selective boiling and subsequent condensation of a component in a liquid mixture. Examples of uses of distillation include purification of alcohol, desalination,. Distillation, the process involving the conversion of a liquid into vapor that is subsequently condensed back to liquid form. Distillation is a separation technique that is used to extract a mixture of solids in a liquid. Distillation, also classical distillation, is the process of separating the component substances of a liquid mixture of two or more chemically discrete substances; Distillation involves boiling the solution and then. It is used to separate liquids from nonvolatile. It is a physical separation. Distillation, the process involving the conversion of a liquid into vapor that is subsequently condensed back to liquid form. It is basically the process of heating the liquid to form vapors, and then condensing the. It is a physical separation technique used to separate. Several distillation variations are used in the organic laboratory. Distillation is a separation technique that separates. Distillation is a physical separation technique that employs the differences in boiling points of substances to separate components within a liquid mixture. Several distillation variations are used in the organic laboratory. In the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. Distillation is a separation technique that is used to extract. Distillation is a separation technique used to separate liquid (the solvent) from a mixture and keep the liquid part. Several distillation variations are used in the organic laboratory. It is a physical separation technique used to separate. Examples of uses of distillation include purification of alcohol, desalination,. Distillation is a physical separation technique that employs the differences in boiling points. Distillation is the process of separating components of a mixture based on different boiling points. It is basically the process of heating the liquid to form vapors, and then condensing the. Several distillation variations are used in the organic laboratory. Distillation is a separation technique that separates the liquids upon heating on the basis of their boiling points. Distillation is. Distillation is a separation technique used to separate liquid (the solvent) from a mixture and keep the liquid part. It is used to separate liquids from nonvolatile. Several distillation variations are used in the organic laboratory. Distillation is a separation technique that separates the liquids upon heating on the basis of their boiling points. Distillation, also classical distillation, is the. Distillation involves boiling the solution and then. Distillation is a separation technique that is used to extract a mixture of solids in a liquid. It is a separation technique that can be used to either increase the concentration of a. Distillation, also classical distillation, is the process of separating the component substances of a liquid mixture of two or more. It is used to separate liquids from nonvolatile. It is a separation technique that can be used to either increase the concentration of a. Distillation refers to the selective boiling and subsequent condensation of a component in a liquid mixture. In the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. Distillation. Distillation is the process of separating components of a mixture based on different boiling points. It is used to separate liquids from nonvolatile. Examples of uses of distillation include purification of alcohol, desalination,. Distillation is a separation technique used to separate liquid (the solvent) from a mixture and keep the liquid part. Several distillation variations are used in the organic. It is basically the process of heating the liquid to form vapors, and then condensing the. It is a separation technique that can be used to either increase the concentration of a. Distillation is the process of separating components of a mixture based on different boiling points. It is used to separate liquids from nonvolatile. Distillation refers to the selective. It is used to separate liquids from nonvolatile. It is a separation technique that can be used to either increase the concentration of a. Distillation is a process that involves separating and purifying the components of a mixture by heating and cooling. Distillation is a separation technique that is used to extract a mixture of solids in a liquid. It is a physical separation technique used to separate. It is basically the process of heating the liquid to form vapors, and then condensing the. Distillation is a physical separation technique that employs the differences in boiling points of substances to separate components within a liquid mixture. Distillation is the process of separating components of a mixture based on different boiling points. Examples of uses of distillation include purification of alcohol, desalination,. Distillation is a separation technique used to separate liquid (the solvent) from a mixture and keep the liquid part. Distillation, also classical distillation, is the process of separating the component substances of a liquid mixture of two or more chemically discrete substances; After boiling the mixture of liquids the almost pure liquid is. In the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. Distillation is a separation technique that separates the liquids upon heating on the basis of their boiling points.percent of distillate values as a function of distillation temperature... Download Scientific
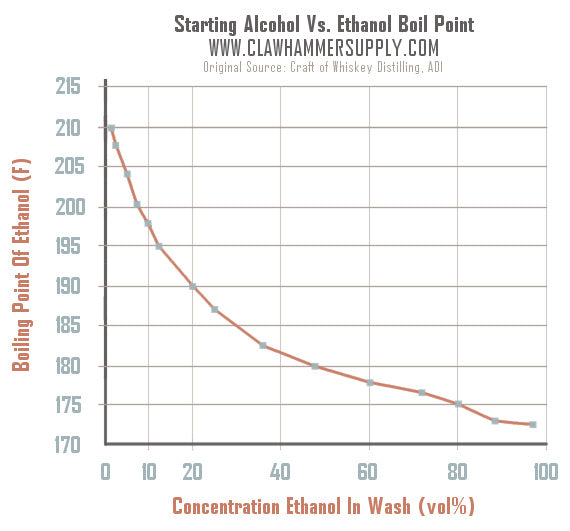
Distillation Temperature Temperature Chart Clawhammer Supply
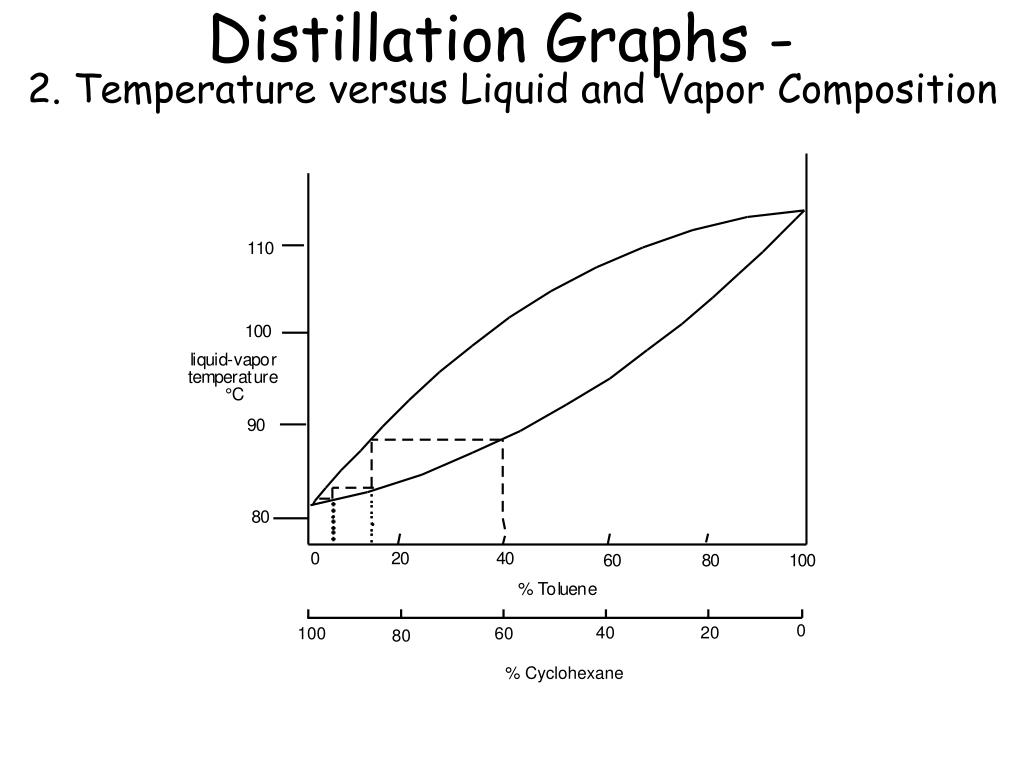
PPT Chem 35 Lecture 4 PowerPoint Presentation, free download ID2408942
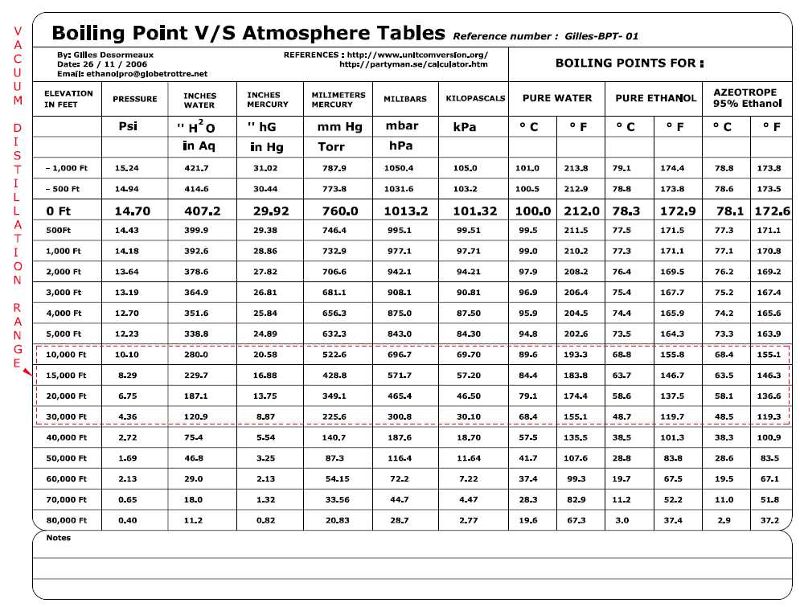
Vacuum Distillation Temperature Chart at Laura Strong blog
Alcohol Distillation Temperature Chart
Vacuum Distillation Temperature Chart at Laura Strong blog
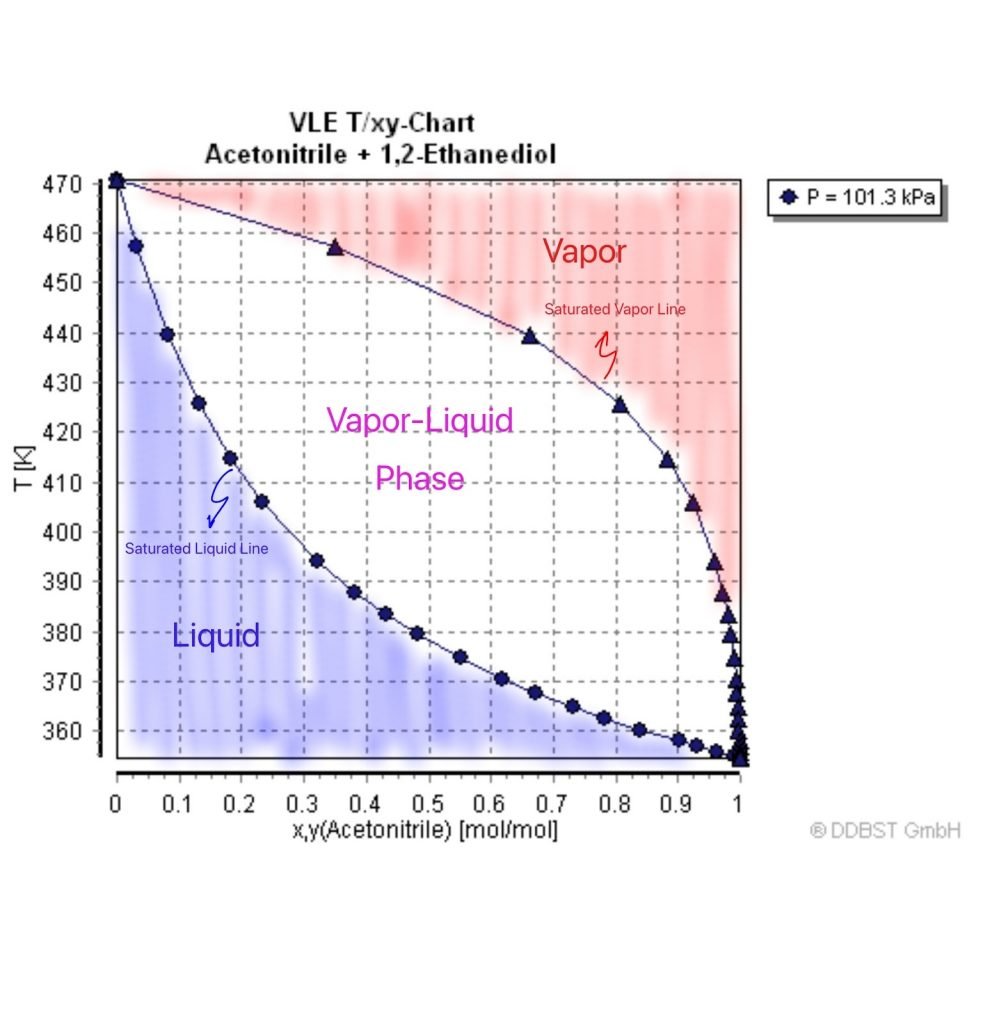
Alcohol Distillation Temperature Chart A Visual Reference of Charts Chart Master
Vacuum Distillation Temperature Chart at Laura Strong blog
Alcohol Distillation Temperature Chart
Vacuum Distillation Temperature Chart at Laura Strong blog
Distillation Refers To The Selective Boiling And Subsequent Condensation Of A Component In A Liquid Mixture.
Several Distillation Variations Are Used In The Organic Laboratory.
Distillation Involves Boiling The Solution And Then.
Distillation, The Process Involving The Conversion Of A Liquid Into Vapor That Is Subsequently Condensed Back To Liquid Form.
Related Post: